Biotechnology has made monumental strides over the past few decades, reshaping the landscape of medicine and offering new hope for treating conditions that were once considered untreatable. One of the most exciting and transformative areas of biotechnology is its role in genetic disease treatment. Genetic disorders, caused by mutations or alterations in the DNA, have historically been difficult to manage, often leading to lifelong challenges or early death for those affected. However, with the advent of powerful biotechnological tools, such as gene therapy, CRISPR-Cas9, RNA-based therapies, and cell-based treatments, the future of genetic disease treatment is brighter than ever.
This article explores how biotechnology is revolutionizing the treatment of genetic diseases, highlighting current advancements, innovative therapies, and the promising horizon of genetic medicine.
- Understanding Genetic Diseases and the Need for Innovative Treatment
Genetic diseases are caused by mutations or defects in a person’s DNA. These mutations can range from a single base pair change to larger structural alterations in the chromosomes. They are often inherited, though some may arise spontaneously. Examples of genetic disorders include:
- Cystic fibrosis: A disease that affects the lungs, digestive system, and other organs, caused by mutations in the CFTR gene.
- Sickle cell anemia: A blood disorder caused by a mutation in the HBB gene, leading to the production of abnormally shaped red blood cells.
- Muscular dystrophy: A group of diseases that cause progressive weakness and loss of muscle mass, caused by mutations in genes responsible for muscle protein production.
- Huntington’s disease: A neurodegenerative disorder caused by an expansion of a DNA sequence in the HTT gene.
These diseases, among many others, often result in severe symptoms, reduced quality of life, and limited treatment options. Traditional treatments have focused on managing symptoms rather than addressing the underlying cause. However, breakthroughs in biotechnology are changing this paradigm, offering the potential for curative therapies that directly target the genetic root cause of these disorders.
- Gene Therapy: A New Era in Treatment
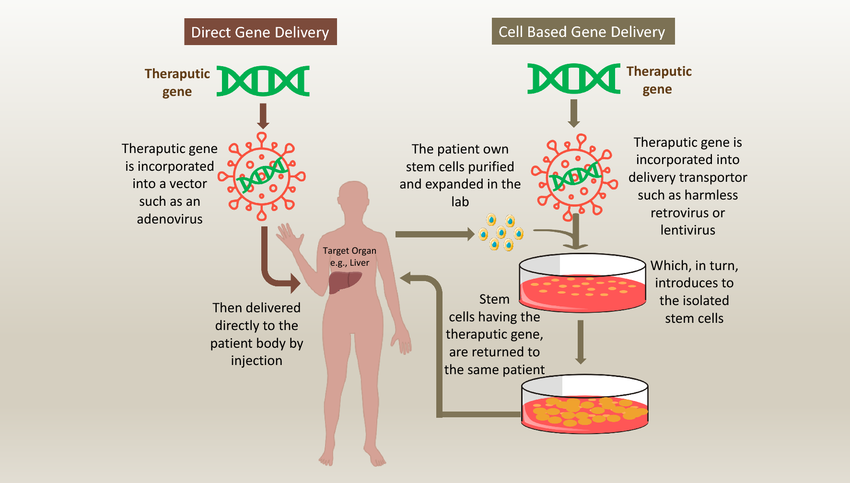
One of the most promising advancements in biotechnology for treating genetic diseases is gene therapy. This approach involves introducing, removing, or altering genetic material within a person’s cells to treat or prevent disease. The goal is to correct or replace faulty genes that are causing disease.
- How Gene Therapy Works
Gene therapy can be performed in two primary ways:
- Somatic gene therapy: This involves altering the genes in the cells of the patient’s body (other than sperm or egg cells). The goal is to treat a specific disease without affecting the patient’s descendants. This method is already being used in clinical trials for various genetic conditions.
- Germline gene therapy: This involves altering the genes in sperm, egg, or embryo cells, which would result in changes that are passed down to future generations. While germline gene therapy holds immense promise, it is still in its early stages, and ethical concerns surrounding its use are significant.
In somatic gene therapy, a healthy copy of the defective gene is delivered into the patient’s cells, often using vectors—viruses that have been modified to carry the healthy gene without causing illness. The healthy gene then takes over the function of the defective one, correcting the disease at its genetic root.
- Examples of Gene Therapy in Action
Gene therapy has already shown promising results in treating various genetic disorders:
- Sickle cell anemia: One of the most groundbreaking applications of gene therapy has been in treating sickle cell anemia. In clinical trials, researchers have successfully used gene therapy to edit the genes of patients’ blood cells, correcting the mutation that causes sickle-shaped red blood cells. These patients have shown significant improvements in their symptoms, with some achieving complete remission from the disease.
- Leber congenital amaurosis (LCA): This is a rare genetic disorder that leads to blindness. In 2017, the FDA approved Luxturna, a gene therapy that delivers a normal copy of the RPE65 gene to the retina, restoring vision in patients with this condition.
- Cystic fibrosis: Gene therapy for cystic fibrosis is still in early stages, but promising results are emerging. Researchers are working to deliver a healthy version of the CFTR gene to patients’ lung cells, with the goal of correcting the mutation that causes the thick mucus buildup characteristic of cystic fibrosis.
- CRISPR-Cas9: Revolutionizing Genetic Editing
Perhaps the most transformative tool in the field of genetic disease treatment is CRISPR-Cas9, a powerful gene-editing technology that allows scientists to precisely alter DNA at specific locations. CRISPR is based on a naturally occurring defense mechanism in bacteria, which they use to cut the DNA of invading viruses. In the lab, scientists adapted this mechanism to target and modify genes in living organisms, including humans.
- How CRISPR-Cas9 Works
CRISPR-Cas9 works by using a small piece of RNA to guide the Cas9 protein to a specific location in the DNA. Once there, Cas9 acts like a pair of molecular scissors, cutting the DNA at the target site. This allows scientists to either remove, replace, or repair the DNA sequence.
The precision and ease of use of CRISPR have made it a game-changer in genetic medicine. With CRISPR, researchers can now directly edit the genes responsible for genetic diseases, offering the potential to correct the mutation at the source.
- CRISPR Applications in Genetic Disease Treatment
CRISPR has shown significant promise in treating a variety of genetic diseases:
- Sickle cell anemia: Researchers have used CRISPR-Cas9 to edit the HBB gene responsible for sickle cell disease. By correcting the mutation in hematopoietic stem cells (which produce blood cells), patients can produce healthy red blood cells. Early clinical trials have shown that this approach can lead to long-term relief from sickle cell symptoms.
- Muscular dystrophy: CRISPR is also being used to treat Duchenne muscular dystrophy, a condition caused by mutations in the DMD gene. Researchers are working on editing the DMD gene in muscle cells, with the goal of restoring the production of dystrophin, a protein necessary for muscle function.
- Huntington’s disease: CRISPR holds potential for treating Huntington’s disease, a neurodegenerative disorder caused by an expansion of the CAG repeat in the HTT gene. By targeting and editing the mutated gene, scientists hope to slow or halt the progression of the disease.
- RNA-Based Therapies: A New Frontier
Another promising avenue of genetic disease treatment is the use of RNA-based therapies. RNA plays a key role in translating genetic information from DNA into proteins, and many genetic diseases result from faulty RNA production. RNA-based therapies aim to correct or replace defective RNA to restore normal protein function.
- RNA Interference and Antisense Oligonucleotides
One RNA-based strategy is RNA interference (RNAi), which involves silencing or “turning off” a faulty gene’s expression. In diseases like amyotrophic lateral sclerosis (ALS) and Huntington’s disease, RNAi can be used to reduce the production of harmful proteins caused by mutated genes.
Another technique is the use of antisense oligonucleotides (ASOs). These are short strands of synthetic RNA that can bind to the RNA produced by a defective gene, preventing the production of abnormal proteins. The FDA recently approved an ASO-based therapy called Spinraza for spinal muscular atrophy (SMA), a genetic disorder that causes muscle weakness. Spinraza works by increasing the production of a protein that is missing or defective in SMA patients.
- Cell-Based Therapies: Replacing or Repairing Damaged Cells
Another major advancement in biotechnology for genetic disease treatment is cell-based therapies. These therapies involve the use of stem cells, gene-edited cells, or modified immune cells to treat genetic disorders.
- Stem cell therapy: Stem cells have the potential to become any type of cell in the body, making them valuable for replacing damaged or defective tissues. In genetic diseases like sickle cell anemia, stem cells can be used to generate healthy red blood cells to replace defective ones.
- CAR-T cell therapy: This involves modifying a patient’s T-cells (immune cells) to better recognize and attack specific diseases, such as cancer. CAR-T cell therapy has shown remarkable success in treating blood cancers like leukemia and lymphoma.
- Challenges and Ethical Considerations
While the potential of biotechnology in genetic disease treatment is immense, several challenges remain. The safety and long-term effects of gene therapies, particularly for germline editing, are still not fully understood. Additionally, access to these therapies is often limited by cost, which raises questions about equity in healthcare.
Ethical concerns also surround the possibility of designer babies, where genetic traits could be selected for non-medical reasons. As gene-editing technologies advance, policymakers will need to address these ethical dilemmas to ensure that genetic therapies are used responsibly and equitably.
- The Future of Genetic Disease Treatment
Despite the challenges, the future of genetic disease treatment looks incredibly promising. Biotechnology is ushering in a new era of precision medicine, where treatments are tailored to an individual’s genetic makeup. As gene-editing technologies like CRISPR, RNA therapies,